39 lewis dot diagram for so3 Wiring Diagram
As predicted by VSEPR theory, its structure belongs to the D 3h point group. The sulfur atom has an oxidation state of +6 and may be assigned a formal charge value as low as 0 (if all three sulfur-oxygen bonds are assumed to be double bonds) or as high as +2 (if the Octet Rule is assumed). [7]
SO3 Hybridization Hybrid Orbitals for SO3 (sulfur trioxide) YouTube
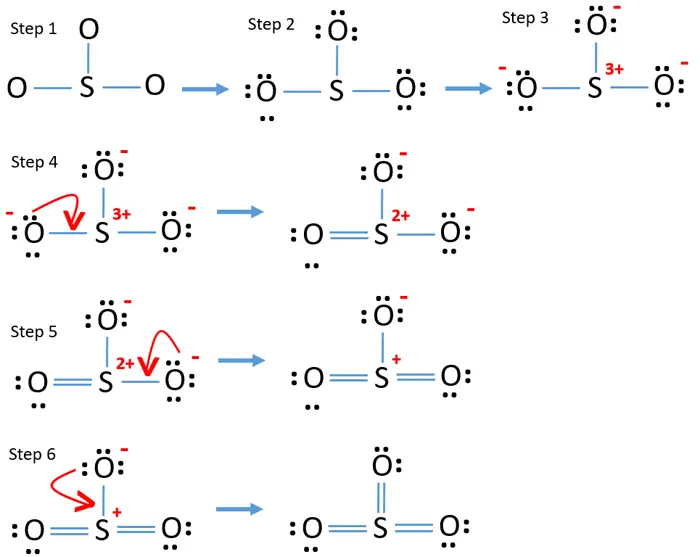
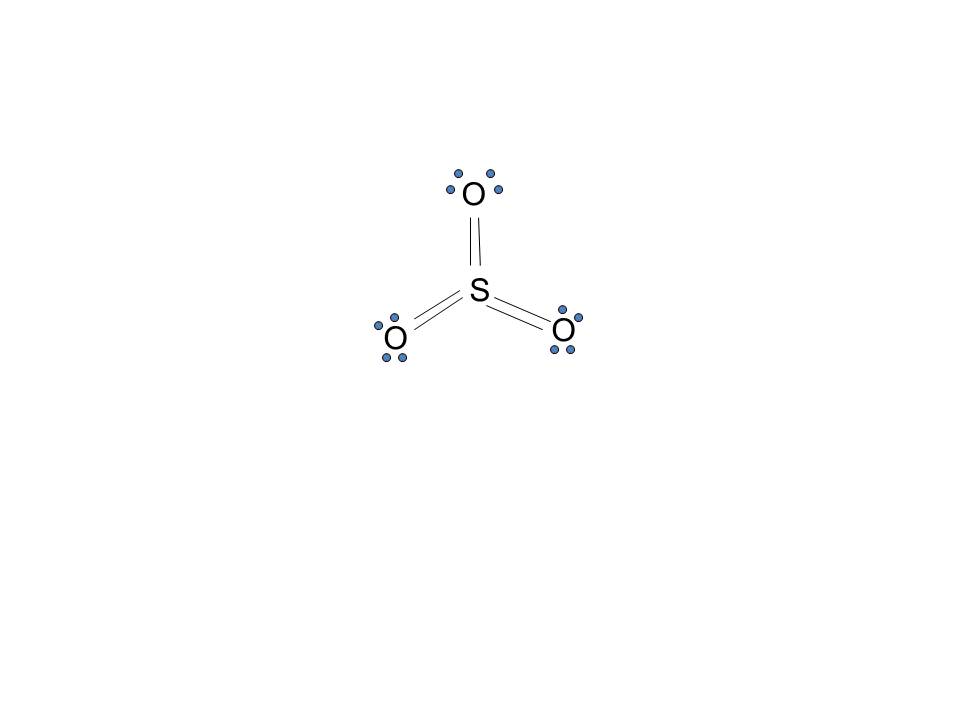
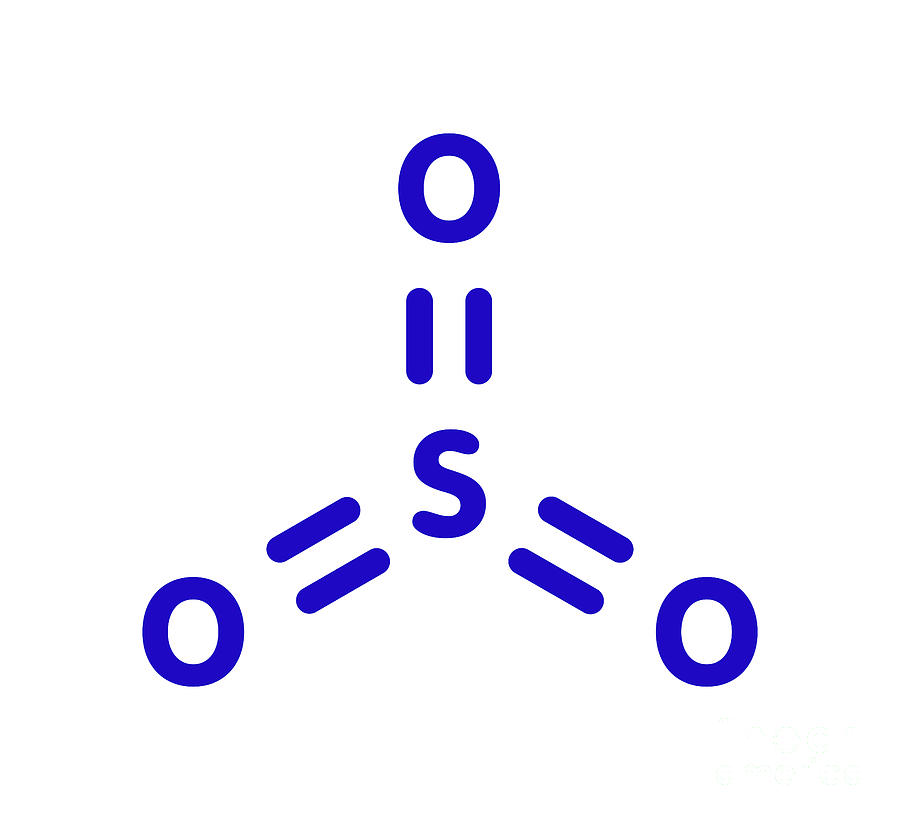
Lewis structure of SO 3 (or Sulfur trioxide) contains three double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center, surrounded by 3 Oxygen atoms (O). The Sulfur atom does not have a lone pair, while all three Oxygen atoms have 2 lone pairs.

Lewis Dot Structure for SO3 (Sulfur trioxide) YouTube
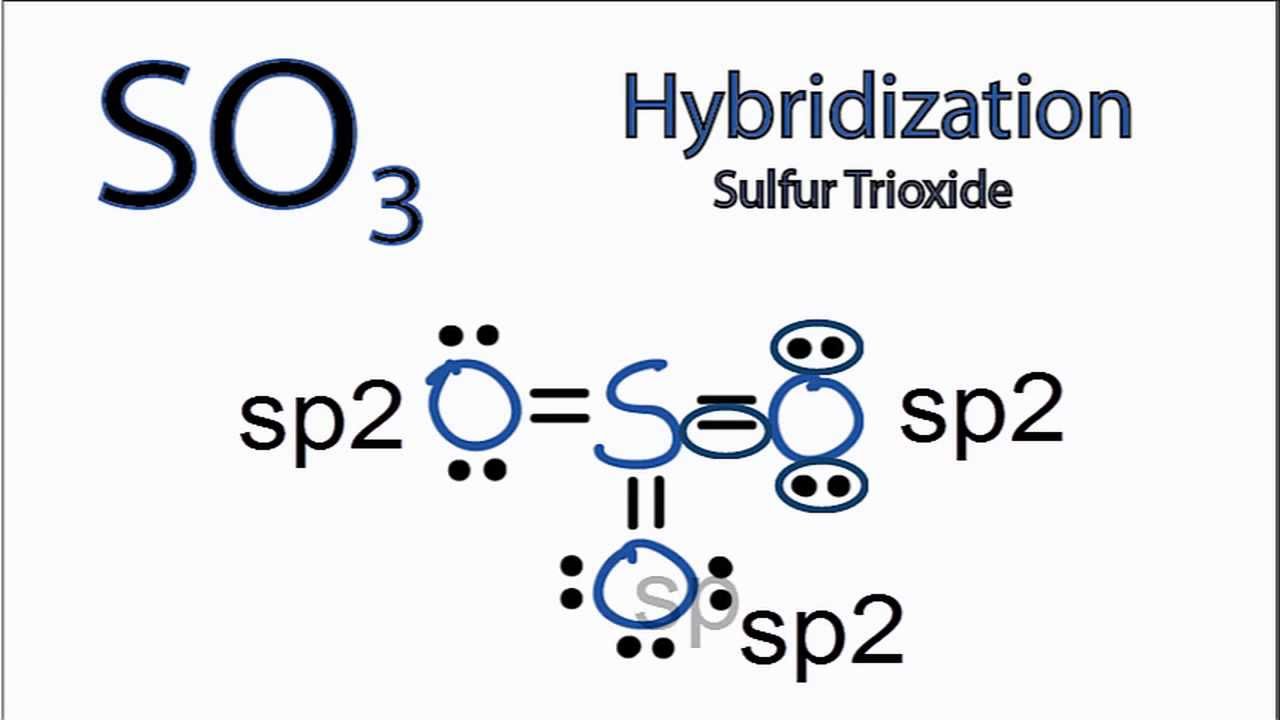
Sulphur trioxide has three resonance structures. But all the structures are equivalent because all the oxygen atoms in SO3 are equivalent. In sulphur trioxide, one sulphur atom and three oxygen atoms are present. In SO 3, sulphur is sp 2 hybridized with a trigonal planar structure and bond angle is 120 0.
Lewis Dot Diagram For Sulfur Wiring Site Resource
Watch on Steps of drawing SO3 lewis structure Step 1: Find the total valence electrons in SO3 molecule In order to find the total valence electrons in SO3 (sulfur trioxide) molecule, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom.

So3 Lewis Structure 2 JalentuGentry
1.3K 357K views 10 years ago SO3 Lewis, Shape, Hybridization, Polarity, and more. A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide). For the SO3.

SO3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
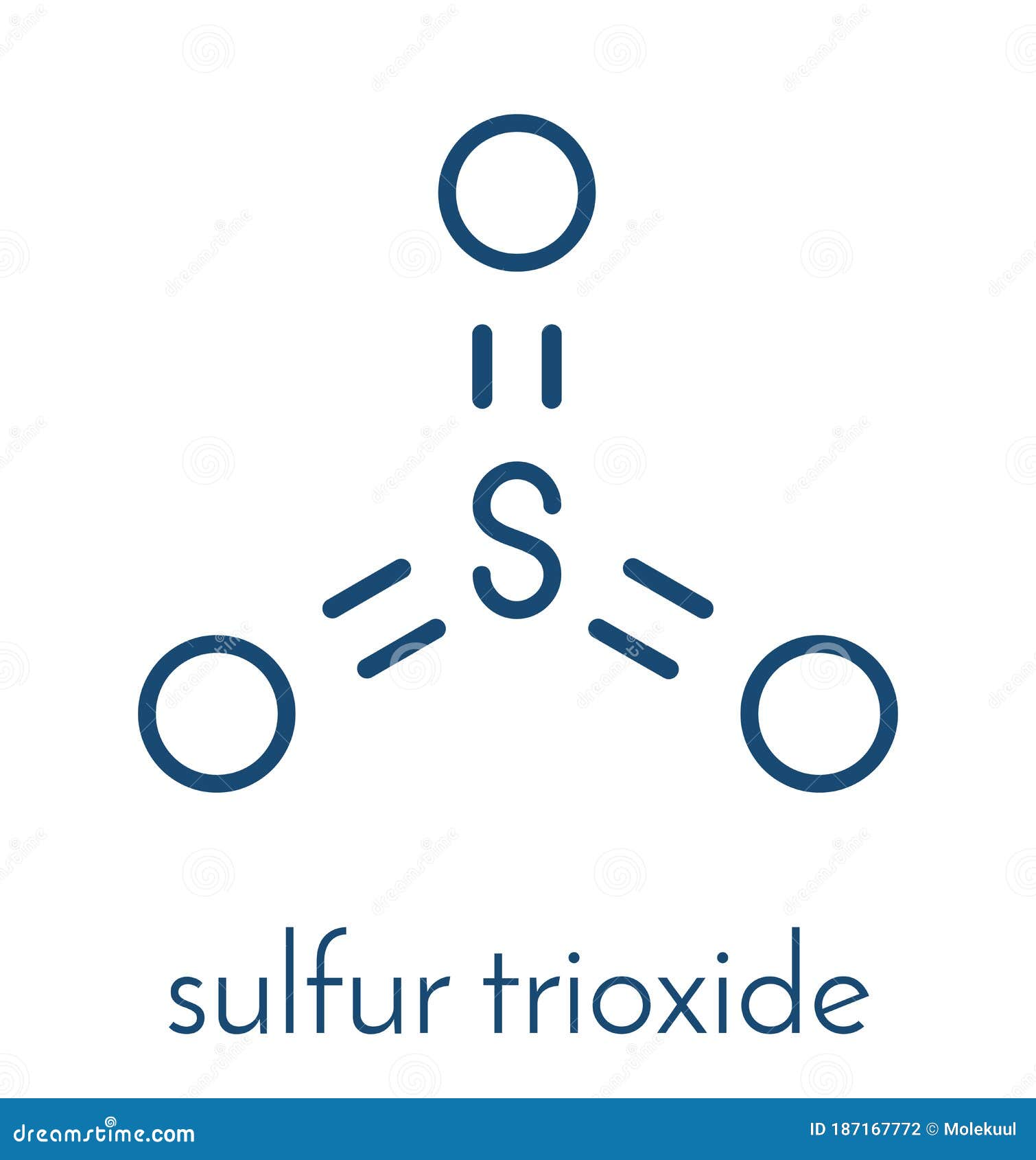
Sulfur Trioxide Pollutant Molecule. Principal Agent In Acid Rain. Skeletal Formula. Vector
The chemical formula for sulfur trioxide is SO3. It is a highly reactive compound that is formed by combining sulfur dioxide and oxygen. SO3 is widely used in the production of sulfuric acid, which is an important industrial chemical. The SO3 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the.

Draw The Lewis Dot Structure For So3 2 slidesharedocs
Video 1.1: Please look at Handout 1: Lewis dot structure technique while watching this video.Note steps 1 and 2 are switched between the video and the handout, but all the rest are the same. Question: Why does sulfur trioxide require resonance structures?In your answer, think about how a Lewis dot structure represents a bond, and the nature of PI bonds.
Sulfur Trioxide Lewis Structure Sulfur Dioxide Resonance PNG, Clipart, Angle, Area, Black And
The SO3 Lewis structure illustrates how the atoms of sulfur trioxide, a molecule composed of one sulfur atom and three oxygen atoms, are arranged. Within the SO 3 Lewis structure, the sulfur atom is bonded to three oxygen atoms through double bonds. Additionally, each oxygen atom has two lone pairs of electrons associated with it.

SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur Trioxide) YouTube
A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number.
Sulfur Trioxide Pollutant Molecule Photograph by Molekuul/science Photo Library
Chemistry Chemistry questions and answers Draw the Lewis structure for the sulfur trioxide (SO_3) molecule. Be sure to include all resonance structures that satisfy the octet rule. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
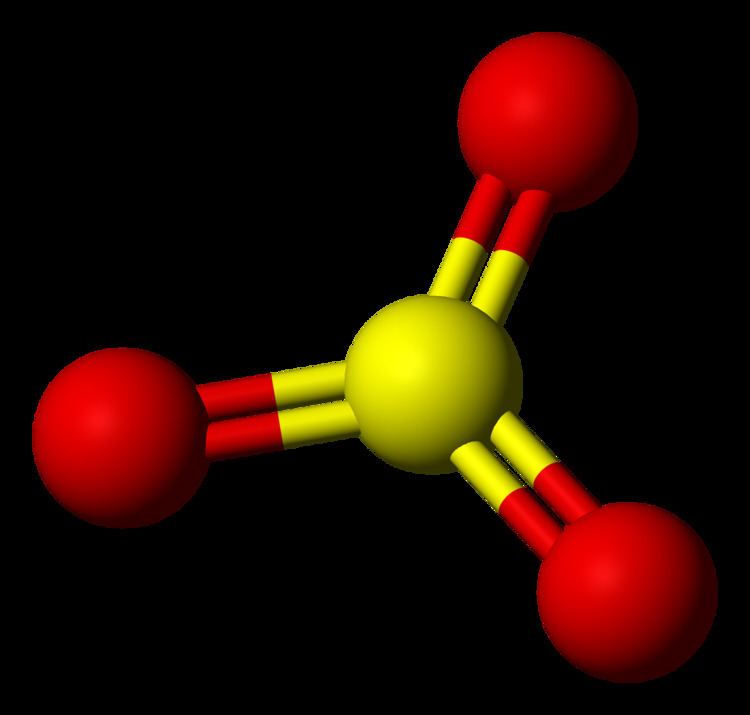
Sulfur trioxide Alchetron, The Free Social Encyclopedia
Lewis structure of SO 3 molecule There are three double bonds around sulfur atom with oxygen atoms in SO molecule. Each oxygen atom has two lone pairs in SO 3 lewis structure. But, there is no lone pair on sulfur atom in SO 3 lewis structure as lewis structure of SO 2. Hybridization of SO 3 molecule All atoms have sp 2 hybridization.

SO3 Molecular Geometry / Shape and Bond Angles (Sulfur Trioxide) YouTube
This chemistry video explains how to draw the Lewis structure of SO3 - Sulfur Trioxide. It discusses the molecular geometry, bond angle, hybridization, and.

Free download Sulfur trioxide Sulfur dioxide Lewis structure Chemistry, text, logo png PNGEgg
A video explanation of how to draw the Lewis Dot Structure for Sulfur Trioxide, along with information about the compound including Formal Charges, Polarity, Hybrid Orbitals, Shape, and.
IC1 Repaso de Examen de Química
2. The problem is that Lewis structures very rarely give a realistic account of the actual bonding situation in a molecule. It is rather good for organic molecules but for molecules like SO3 it is not suitable. Fact is that all S − O-bonds in SO3 are the same and they are neither typical single nor double bonds.
Sulfur Trioxide Sulfur Dioxide Lewis Structure Oleum, PNG, 1200x1254px, Sulfur Trioxide, Area
Lewis Dot of Sulfur Trioxide. SO 3. Back: 70 More Lewis Dot Structures. S does not follow the octet rule. It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. NOT IN THIS MOLECULE.