
SiH4 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
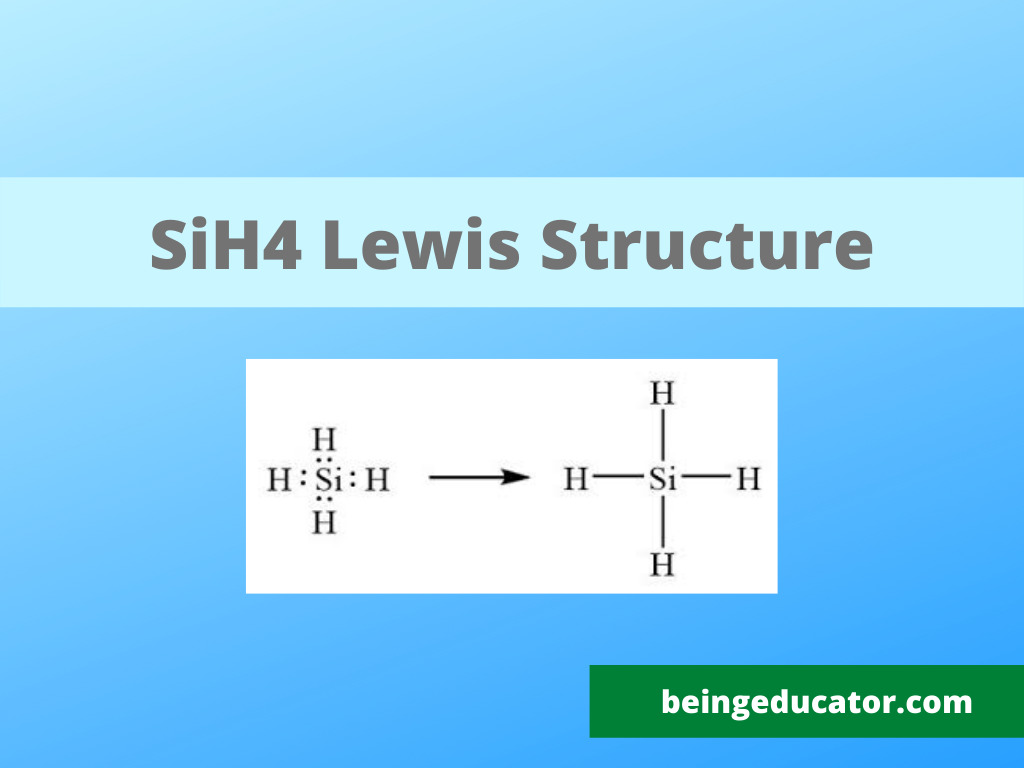
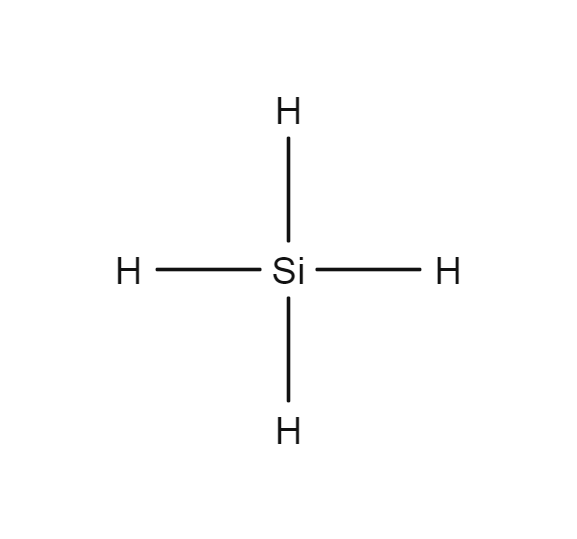
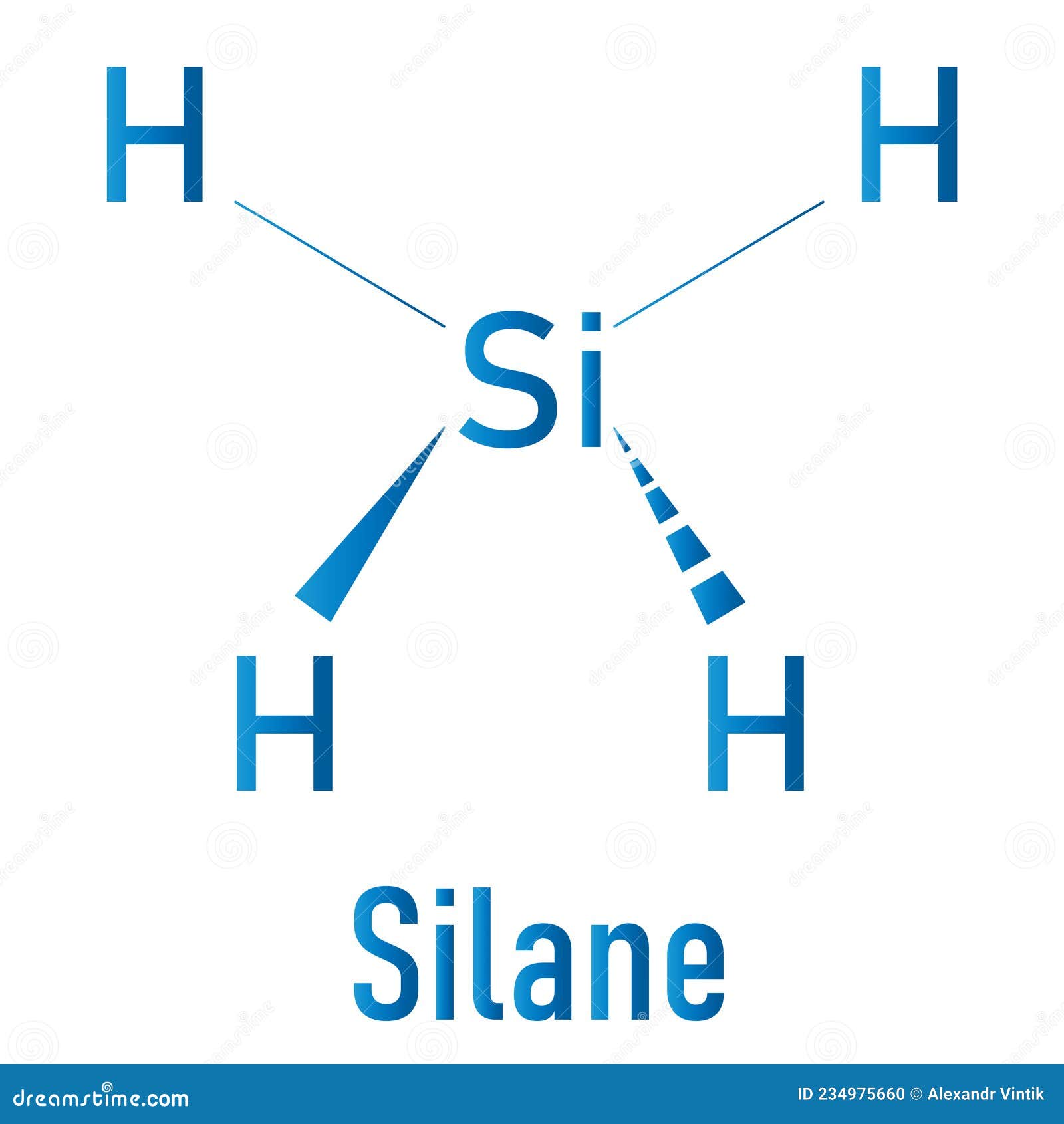
SiH4 lewis structure has a Silicon atom (Si) at the center which is surrounded by four Hydrogen atoms (H). There are 4 single bonds between the Silicon atom (Si) and each Hydrogen atom (H).
Lewis Structure, Hybridization, Polarity and Molecular Geometry of SiH4
Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry for silane (SiH4).

Solved SiH4 1) Silane Lewis structure Electron pair geometry
By Darshana Fendarkar In this article,"SiH4 Lewis structure", different facts like Lewis structure drawing, formal charge calculation, hybridization, structure with some detailed explanations are described below. SiH4 is also called silane or monosilane, it is a colorless flammable and poisonous gas with a strong pungent odor.
Silane Lewis Structure
Monosilane (SiH4) is far less well behaved than its carbon analogue methane (CH4). It is a colourless gas that is industrially relevant as a source of elemental silicon, but its pyrophoric and.

Silane (SiH4) molecule. Skeletal formula Stock Vector Image & Art Alamy
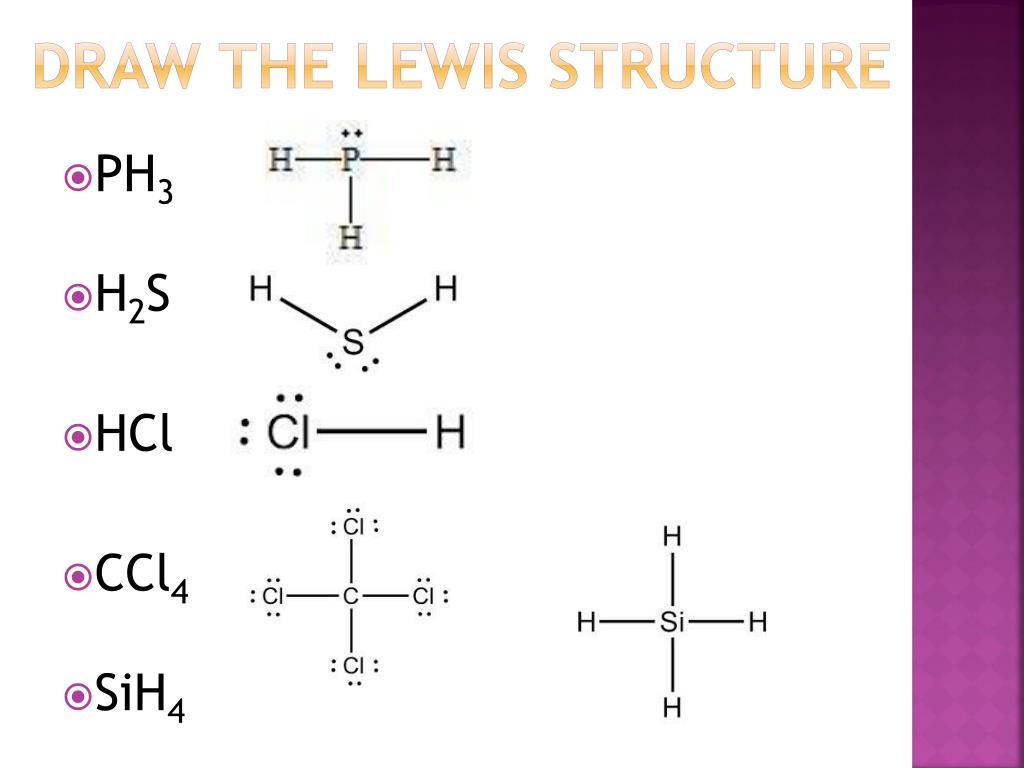
The Lewis structure indicates that each [latex]\ce{Cl}[/latex]. {CCl4}[/latex] (carbon tetrachloride) and silicon in [latex]\ce{SiH4}[/latex] (silane). Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule. The transition elements and inner transition elements also do not follow the octet rule:

Is SiH4 Polar or Nonpolar? (Silicon Tetrahydride) YouTube
It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B-F single bonds. This suggests the best Lewis structure has three B-F single bonds and an electron deficient boron.

Silicon(IV) iodide, 99.999 (metals basis), Thermo Scientific Chemicals
A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrafluoride).For the SiH4 structure use the periodic table to find the tota.

como se forma el sih4 a partir de sus atomos utilizando la estructura
Chemistry 101A Topic F: Molecular Structure 9: Basic Concepts of Covalent Bonding 9.3: Drawing Lewis Structures

Is SiH4 Polar or Nonpolar? Techiescientist
In the SiH 4 Lewis structure, there are four single bonds around the silicon atom, with four hydrogen atoms attached to it, and none of the atoms has a lone pair. Contents Steps #1 Draw a rough sketch of the structure #2 Next, indicate lone pairs on the atoms External links Steps To properly draw the SiH 4 Lewis structure, follow these steps:

Crystal structure of SiH4 at 72 GPa having P4/nbm symmetry. Si atoms
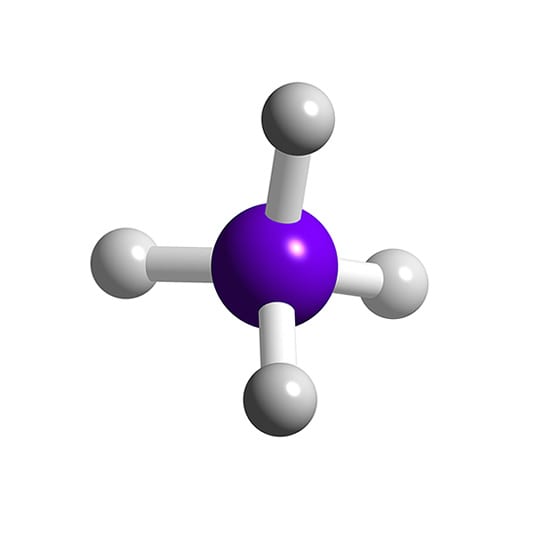
An explanation of the molecular geometry for the SiH4 (Silicon Tetrahydride (Silane) including a description of the SiH4 bond angles. The electron geometry f.
SiH4 Silane
So that is the SiH4 Lewis structure. This is Dr. B., and thanks for watching. Search our 100 + Lewis Structures (Opens New Window) See the Big List of Lewis Structures : Frequently Tested Lewis Structures Basic CH 4, NH 3, C 2 H 4, O 2, N 2 Intermediate O 3, BBr 3, I 3-, BrF 5, NO
8+ ford escape air conditioning diagram SenoTybalt
SiH4 Lewis Structure - How to Draw the Lewis Structure for SiH4 (Silicon Tetrahydride) Watch on So from the above diagram we have come to know that the SiH4 molecule has four Si-H bonds. Now in the next step we have to check whether these four Si-H bonds are polar or nonpolar. And we also have to check the molecular geometry of SiH4.
PPT Covalent Bonds PowerPoint Presentation, free download ID3048466
Draw the Lewis structure for SiH4. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons. Include all hydrogen atoms. To change the symbol of an atom, double-click on the atom and enter the letter of the new atom. PART B Draw the Lewis structure for CO.

SiH4 Lewis Structure (Silicon Tetrahydride) YouTube
SiH4 Lewis Structure Lewis Structure is a two-dimensional diagrammatic approach towards finding the nature of chemical bonding present inside any given molecule. Here, we use dot notations to represent the electrons, and hence this is also known as the electron-dot structure.
[Solved] Draw the Lewis structure for SiH 4 in the window below and
Structure Chemical Safety Laboratory Chemical Safety Summary (LCSS) Datasheet Molecular Formula H4Si SiH4 Synonyms 5J076063R1 7803-62-5 silane Silicane SiH4 View More. Molecular Weight 32.117 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates Create: 2004-09-16 Modify: 2023-12-30 Description
Silane SiH4 Molecule. Skeletal Formula Stock Vector Illustration of
A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrahydride).For the SiH4 structure use the periodic table to find the total.