
SOLVED25 psi of ethylene gas (C2H2) is reacted with oxygen to produce
3.(4 points) A student is given a 6.216 g mixture of iron filings, calcium chloride and sand. He separates the mixture and recovers 2.524 g of iron, 1.932 g of sand and 1.523 g of calcium chloride. Calculate the percentage of each component he recovered from the original mixture and the percent of material he lost during the separation process.
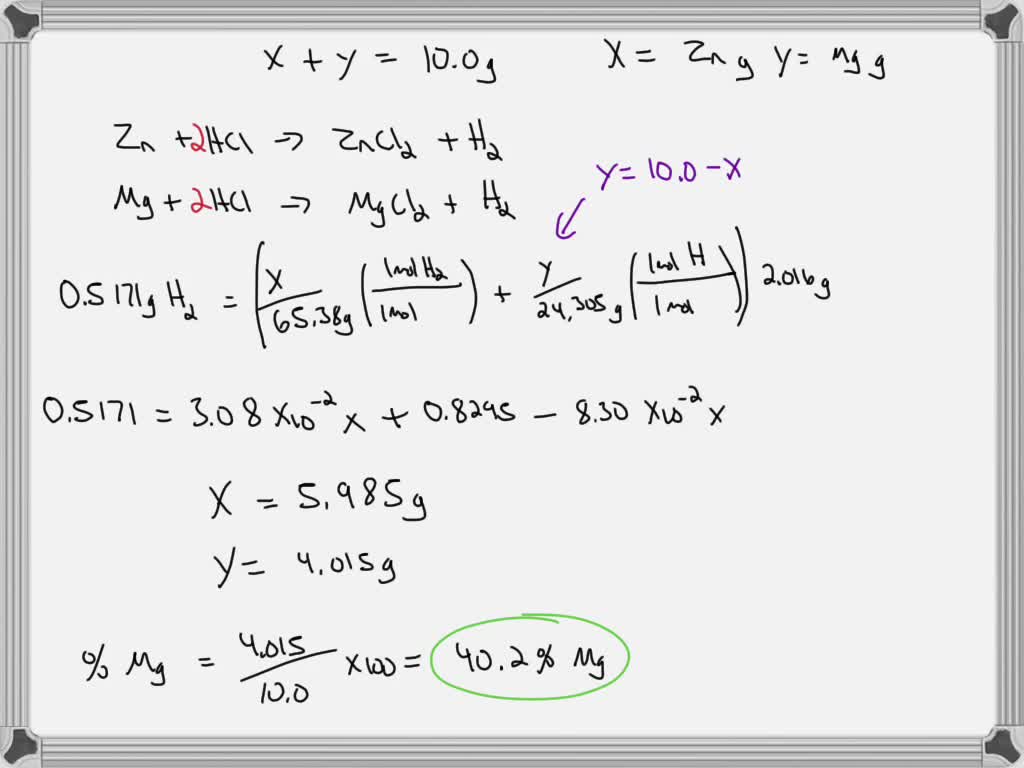
SOLVED A 20.00 g mixture of magnesium and zinc metal reacting with
Mixture problems involve combining two or more things and determining some characteristic of either the ingredients or the resulting mixture. For example, we might want to know how much water to add to dilute a saline solution, or we might want to determine the percentage of concentrate in a jug of orange juice. We can use fractions, ratios, or percentages to describe quantities in mixtures.

Solved A 9.780g gaseous mixture contains ethane (C2H6) and
A 26.16 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. The reaction mixture is then reacted with 304 mL of 1.65 M barium chloride to produce the maximum possible amount of barium sulfate. Determine the percent sodium by mass in the original mixture. mass percent Na=
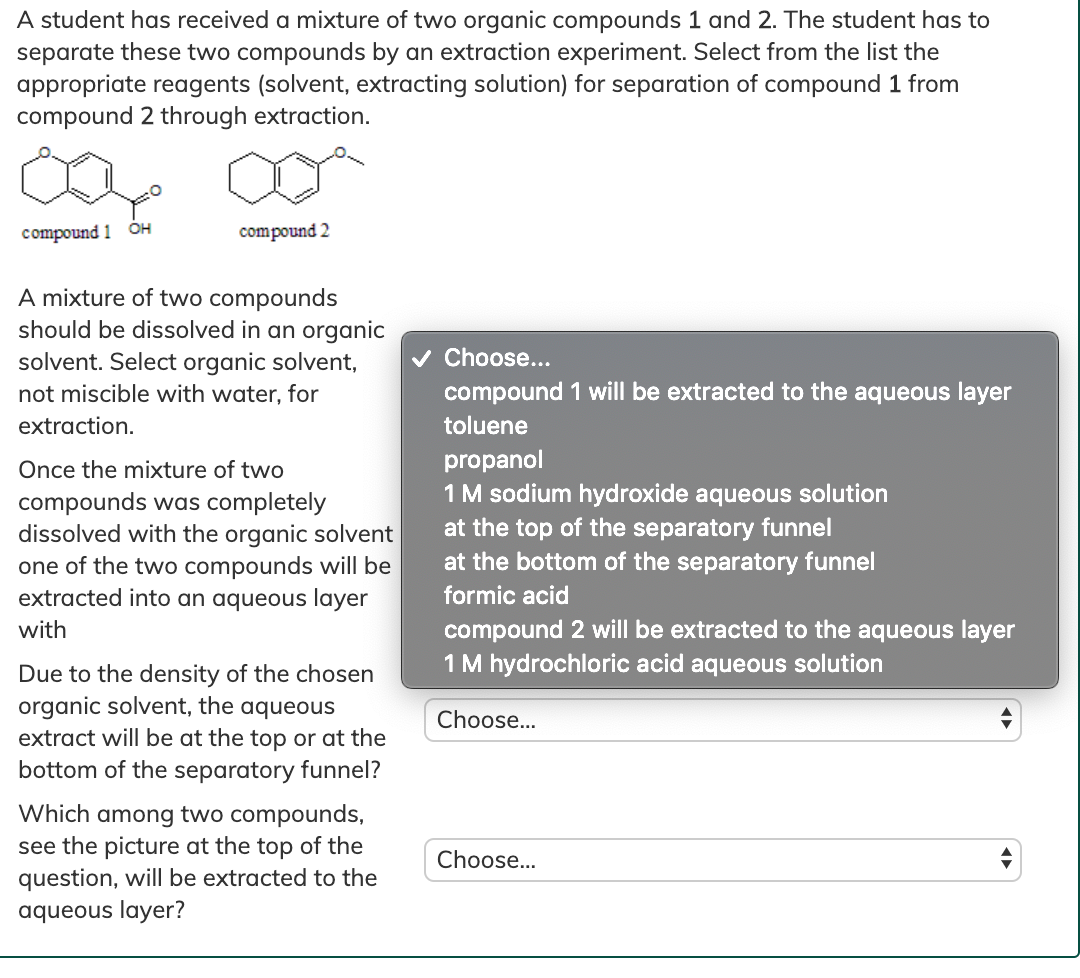
Solved A student has received a mixture of two organic
Distillation is a purification technique for a liquid or a mixture of liquids. We utilize the difference in boiling points of liquids as a basis of separation. The core of a distillation process, is selective evaporation and condensation of particular components. Our overall goal is to evaporate and condense only one component from a mixture.

Buckley's Cough Syrup for Cough and Congestion Relief, Original Mixture
The creation of Buckley's Original Mixture was a classic case of recognizing a good thing. When pharmacist William Knapp Buckley took over a Toronto drugstore in 1919, he discovered several natural ingredients used in the treatment of coughs and colds. W.K. recognized the ingredients' merits and combined them to create a unique and effective.
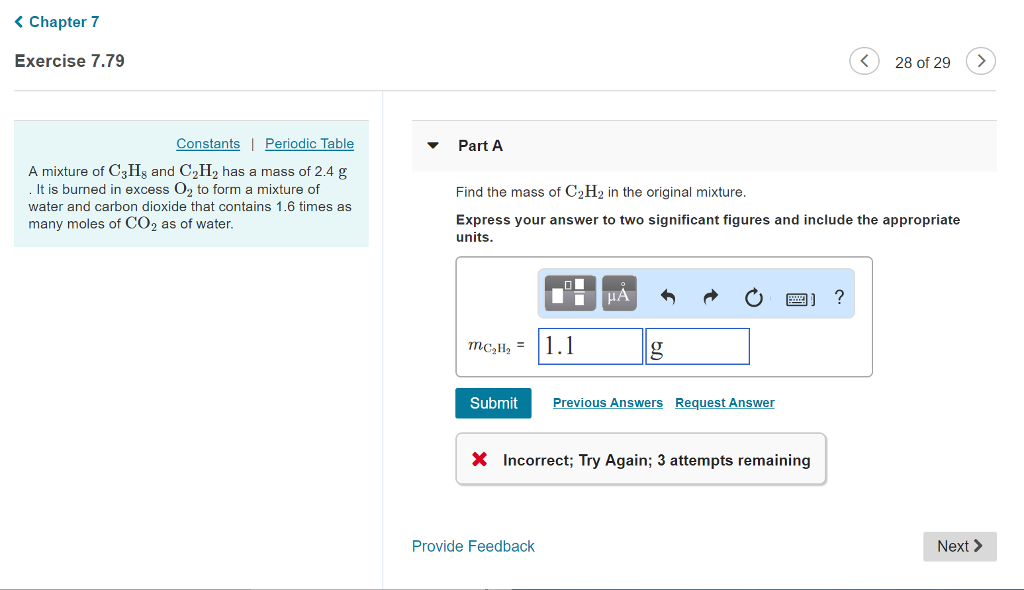
Solved A mixture of C3H8 and C2H2 has a mass of 2.4 g . It
Molar mass of a chemical is the mass, in grams, one mole that that chemical has. And it usually is in units of grams per mole, or g/mol. So here the molar mass of potassium chloride is 74.55 g/mol, which means that one mole of potassium chloride, 6.022x10^ (23) potassium chlorides, has a mass of 74.55 grams. Hope that helps.
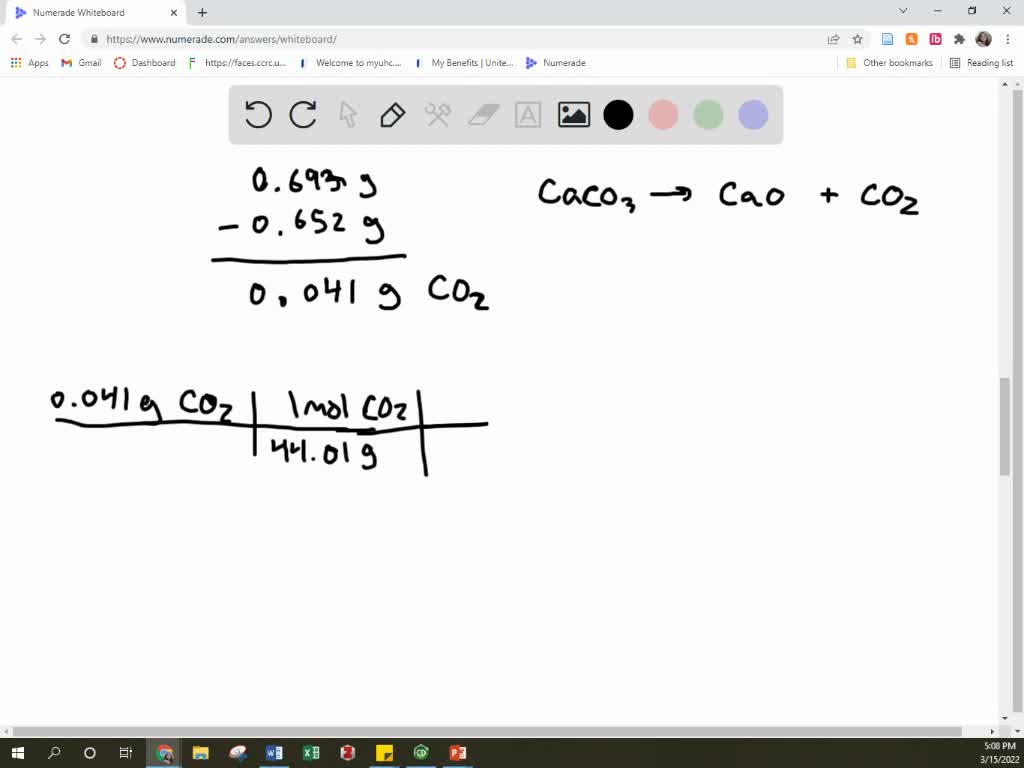
SOLVED A mixture of CaCO3 and CaO weighing 0.693 g was heated to
Assuming all the $\ce{KClO3}$ decomposed to $\ce{KCl}$ and $\ce{O2}$, calculate the mass percent of $\ce{KClO3}$ in the original mixture." I took the amount of $\ce{O2}$, converted it to moles $\ce{O2}$, then divided it to moles of just O, then divived by 3, bcause for every 3 O (for the 3 in the $\ce{KClO3}$) there is a mole of $\ce{KClO3}$ so.

SOLVEDThe amount of ozone, O3, in a mixture of gases can be determined
When the mixture is dissolved in water and an excess of silver nitrate is added, all the chloride ions associated with the original mixture are precipitated as insoluble silver chloride (AgCl). The mass of the silver chloride is found to be 2.1476 g. Calculate the mass percentages of sodium chloride and potassium chloride in the original mixture.
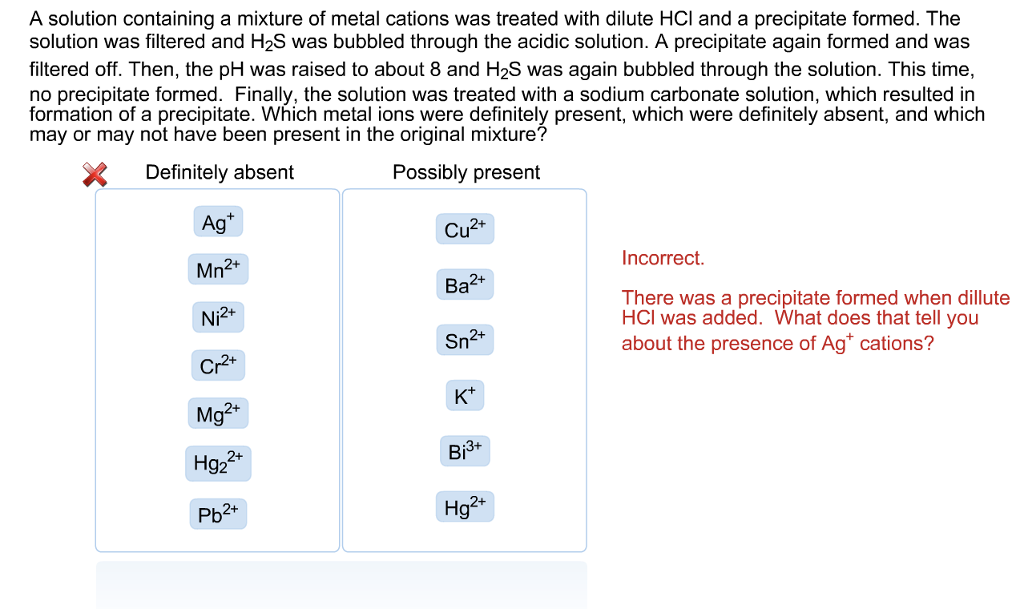
Solved A solution containing a mixture of metal cations was
A heterogeneous mixture consists of two or more phases. When oil and water are combined, they do not mix evenly, but instead form two separate layers. Each of the layers is called a phase. Figure 9.1.1 9.1. 1 : Oil and water do not mix, instead forming two distinct layers called phases. The oil phase is less dense than the water phase, and so.

Buckley's Original Nighttime Mixture 100mL (3.4oz)
A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions and colloids. [2] [3] Mixtures are one product of mechanically blending or mixing chemical substances such as elements and compounds, without chemical bonding or other chemical change, so that.

Mixture 1kg
Buckley is the only product I truly have full trust and confidence in. It work right away whenever there is a need to use for mild congestion or cough. Its easy to digest even with the mild bad taste of it. 2.0/5. Value / Valeur. Quality / Qualité. Effectiveness / Efficacité.
Buckley's Original Mixture Cough & Congestion 200ml London Drugs
Peterson's pipe tobaccos follow in the same spirit of "outstanding craftsmanship" with an eye to using the best tobacco leaf to create "unique and distinctive blends that emphasize the character and heritage of our name.". The adherence to heritage definitely went into the creation of the 1865 Mixture, named for the year the company.

SOLVEDA mixture contains only CuCl2 and FeCl3. A 0.7391 g sample of
Our original mixture has 350 oz. Our mixture ratio was calculate for 100% - we may think of it as 100 oz. 1 000 oz/100 oz = 10 - we need 10 times more ingredients. We're using the ratio's nominators: Pineapple juice: 28.57 × 10 = 285.7 oz; Apple juice: 57.14 × 10 = 571.4 oz;
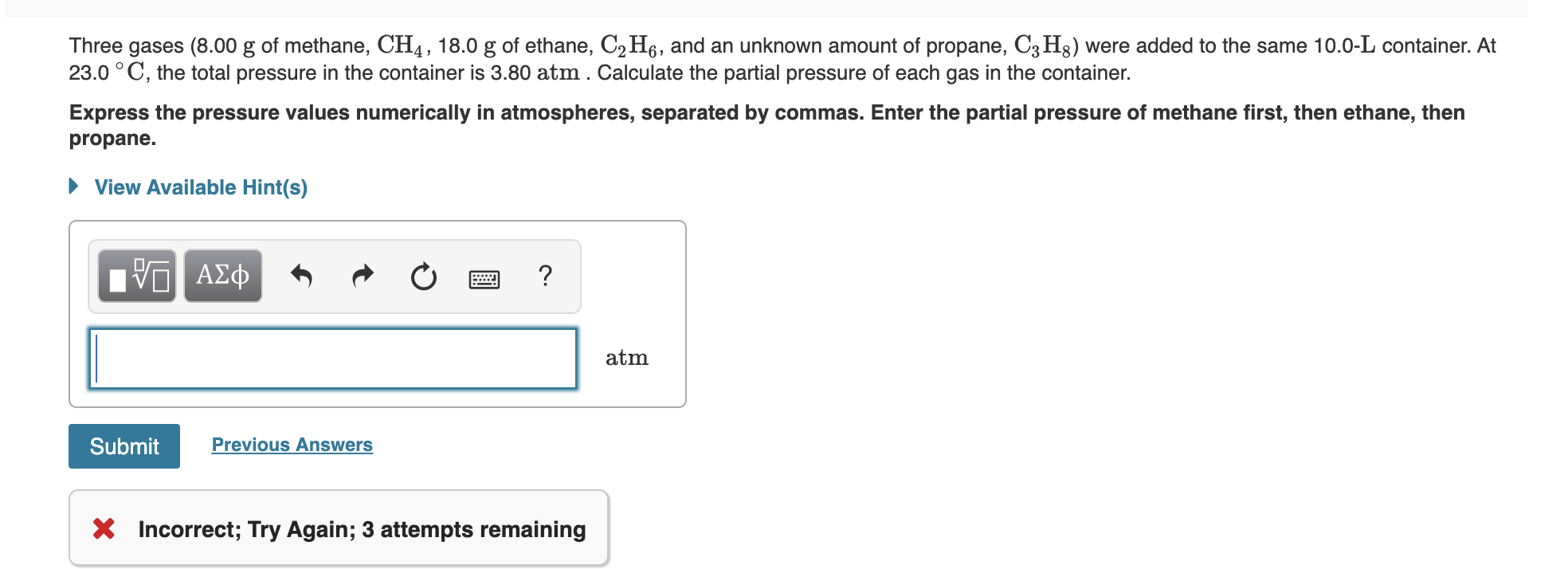
Answered Three gases (8.00 g of methane, CH4,… bartleby
The original "Original" is no longer produced due to the loss of an English blending component. This burley based blend has a distinct blend of two aromatics in the subtlest proportions.. Original Mixture is not for the faint of heart or novice pipe smoker. 4 people found this review helpful. Please login to upvote this review. Reviewed.

Vegetable Mixture Free Stock Photo Public Domain Pictures
Wet the paper with deionized water in order to hold it in place in the funnel. 6. Add 10 - 15 mL of deionized water to the dish and stir for five (5) minutes. Using the stirring rod to direct the slurry, pour the mixture into the filter always keeping the liquid level below the top edge of the filter paper.

A 0.9157 g mixture of CaBr 2 and NaBr is dissolved in water, and AgNO 3
Like a mixture, solutions can be separated into its original components. However, unlike mixtures, solutions can be separated by evaporation. For example: the water and salt solution will evaporate as the solution is heated. The water will change from liquid to gas as the water-salt solution begins to boil, leaving only the salt behind.