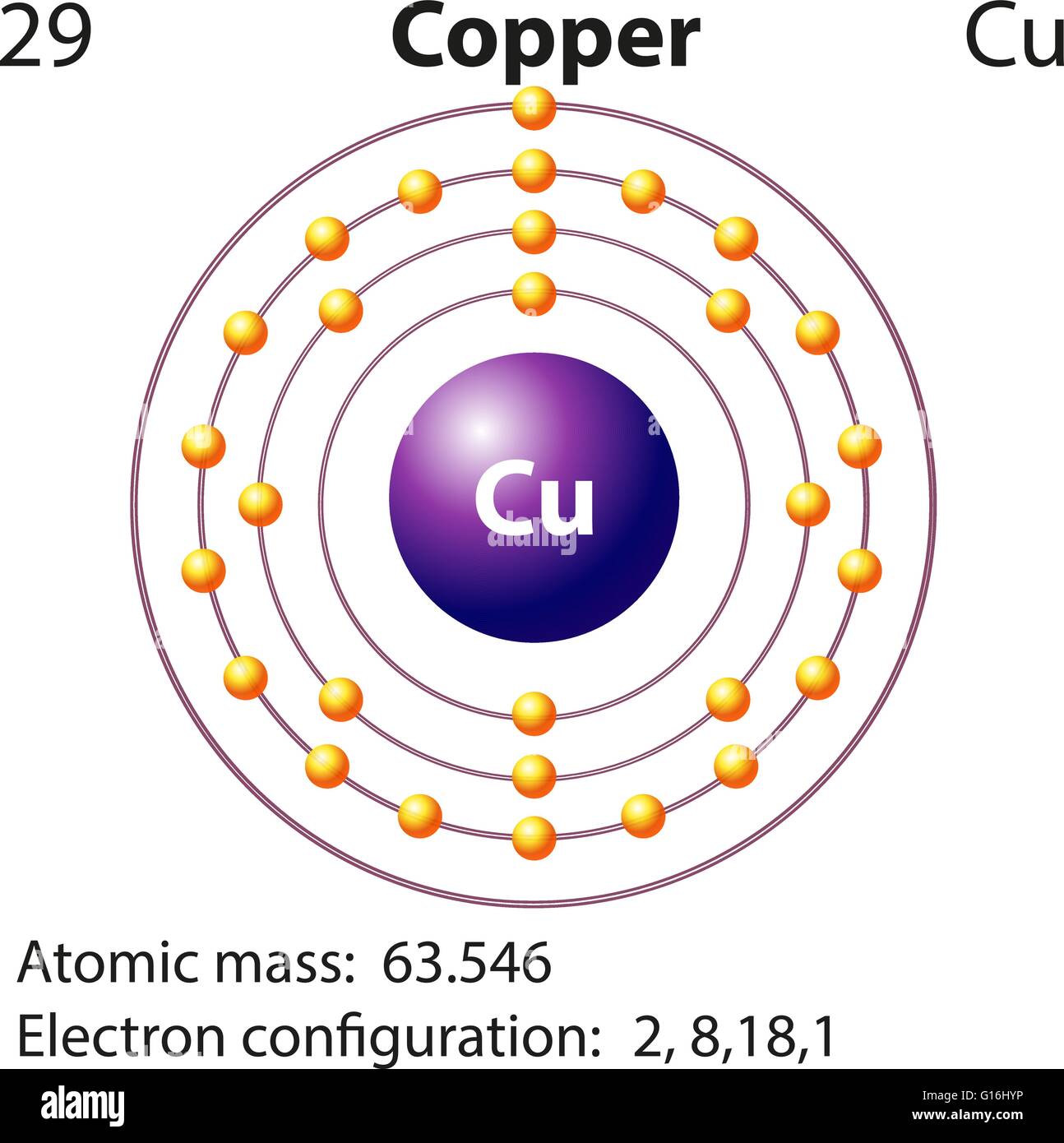
Lewis Dot Diagram For Copper Drivenheisenberg
In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom (there are 29 electrons). Once we have the configuration for Cu, the ions are simple. When we write the configuration we'll put all 29 electrons in orbitals around the nucleus of the Copper atom.
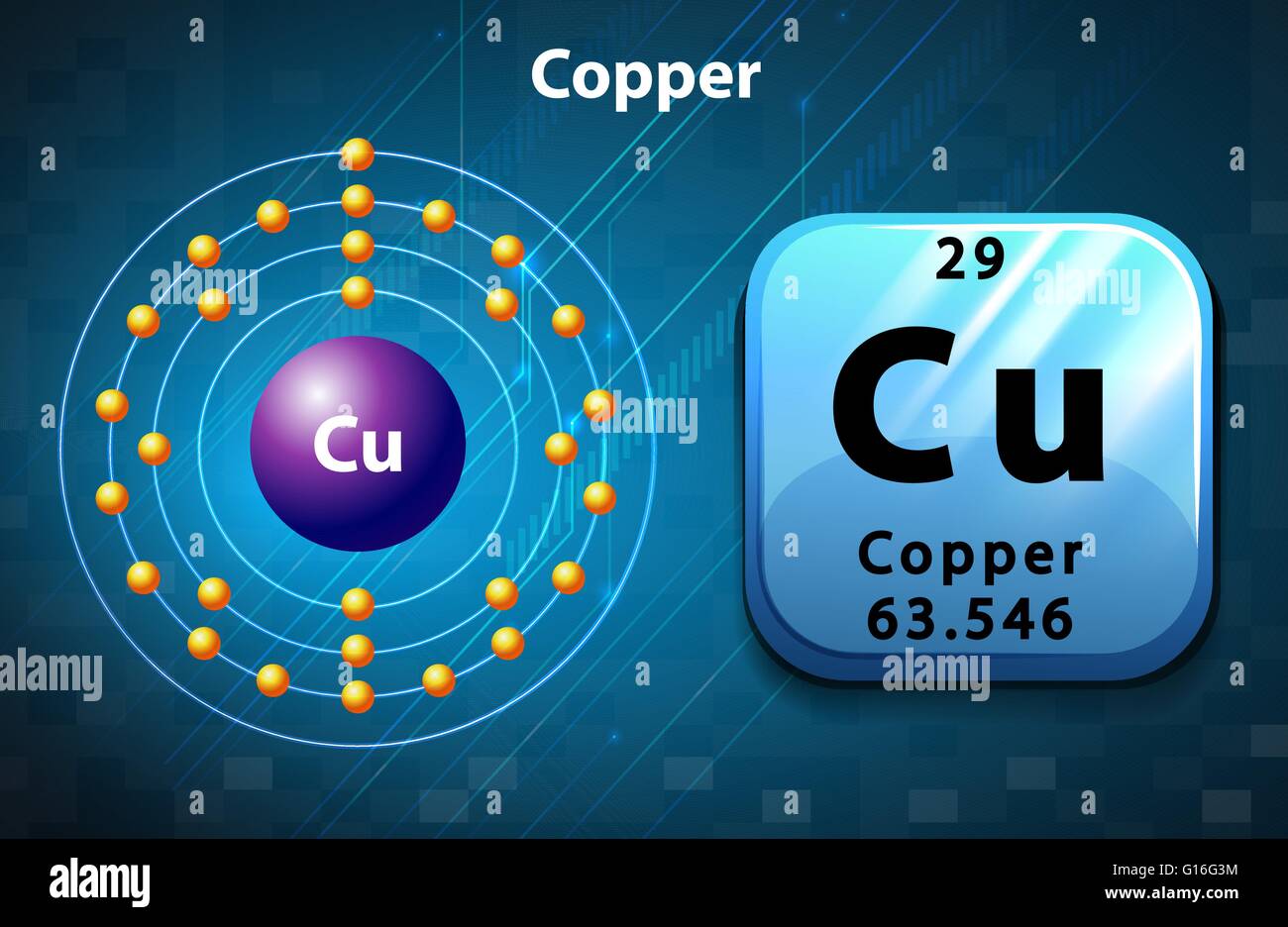
Copper atom hires stock photography and images Alamy
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. Electron configuration of Copper (Cu) [Ar] 3d 10 4s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1: 2, 8, 18, 1: 30: Electron configuration of Zinc (Zn) [Ar] 3d 10 4s 2: 1s 2 2s 2 2p 6 3s 2.

Figure 12 from Effects of the guide field on electron distribution functions in the diffusion
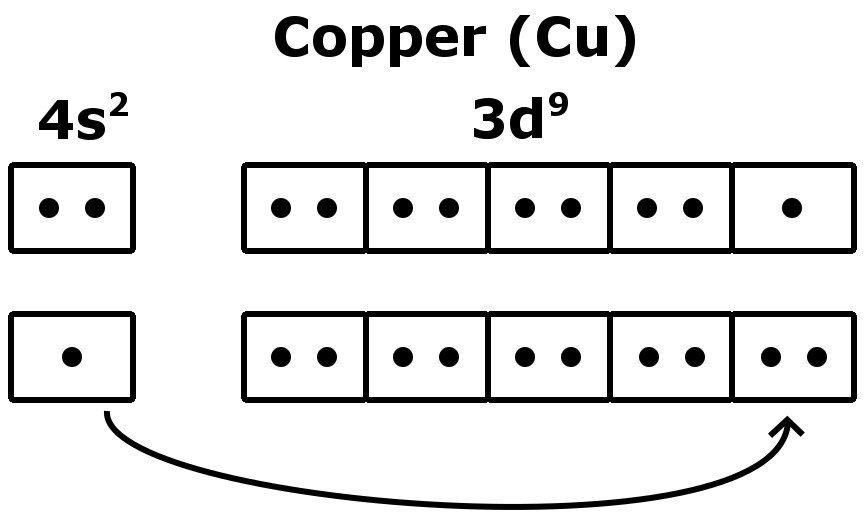
The given electronic configuration, 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s¹, represents the electron distribution of the element with atomic number 29, which is copper (Cu). Copper's electronic configuration is exceptional because it has one electron in the 4s orbital and ten electrons in the 3d orbital, deviating from the expected filling order due to energy-level considerations.
Electron Configuration Of Copper In Ground State
The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:
What is Electricity? SparkFun Learn
The orbital interaction between Cu 6 core and ligands (SR) 6 significantly change the electron distribution of copper core and made the copper cluster have the special optical and electronical characters. • The UV-visible absorption peaks mainly came from the electron excitation from metal core Cu 6 and 6 S atoms to the ligands (SR) 6.
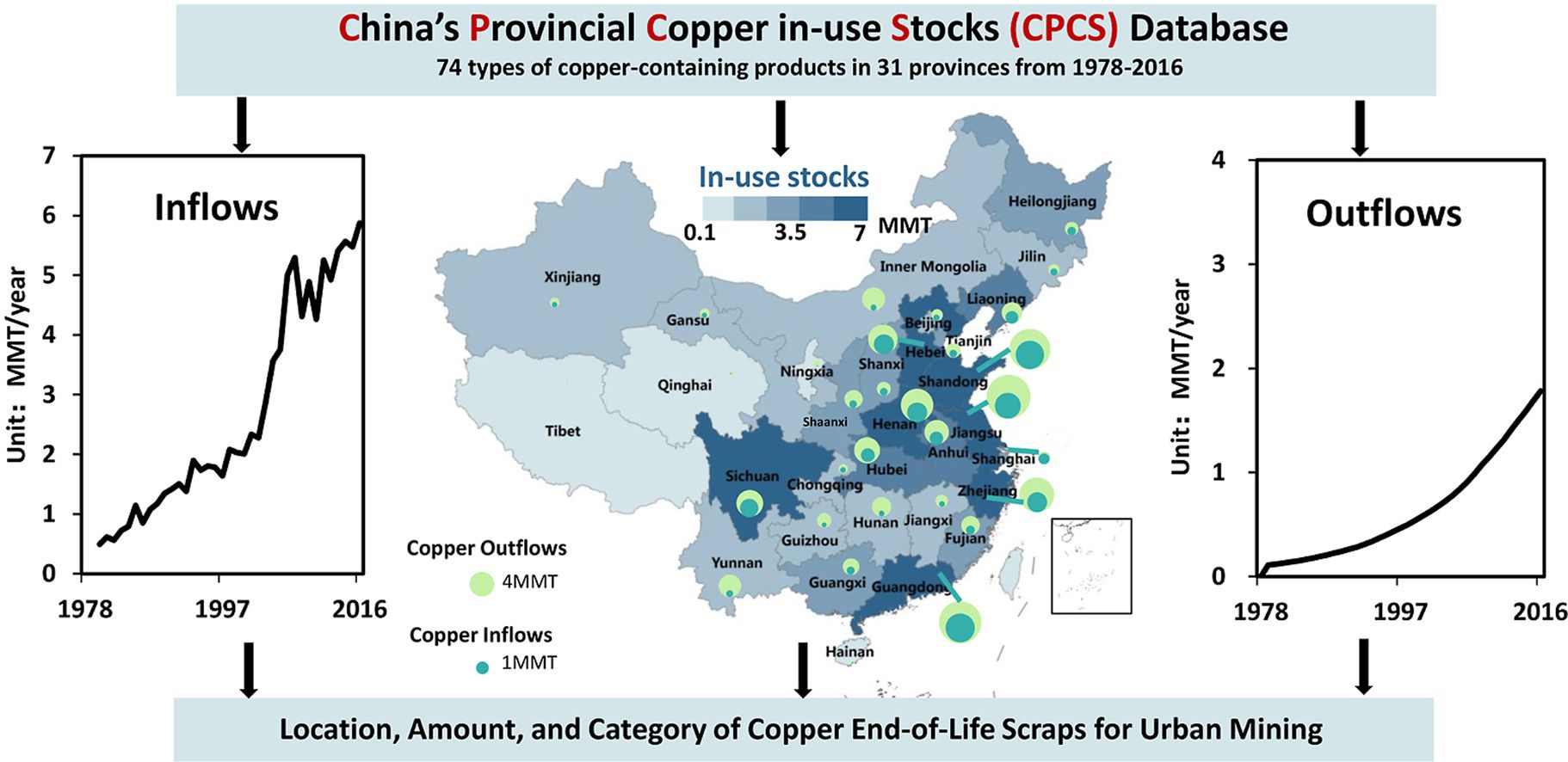
Spatial distribution of copper inuse stocks and flows in China 19782016
atomic physics (or other physical structure) in [1] For example, the electron configuration of the , meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals.

Copper absorption and distribution. Copper values indicate the average... Download Scientific
The arrangement of electrons in copper in specific rules in different orbits and orbitals is called the electron configuration of copper. The electron configuration of copper is [ Ar] 3d 10 4s 1 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)
How To Find A Electron Configuration For Copper Dynamic Periodic Table of Elements and Chemistry
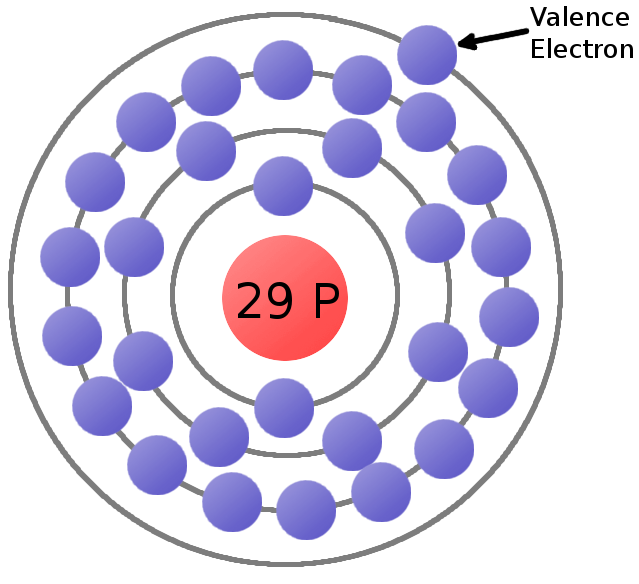
The electron distribution in copper spans across the K, L, M, and N shells, with valence electrons located in the 4s and 3d orbitals. Copper's valency can be either +1 or +2, with the valence electron participating in chemical bonding.

Electron distribution function as seen by the two electron detectors ,... Download Scientific
Electron configuration of Copper is [Ar] 3d10 4s1. Possible oxidation states are +1,2. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological.

How to Write the Electron Configuration for Copper YouTube
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.

Schematic of a copper atom, showing the four concentric energy levels... Download Scientific
Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, Visit BYJU'S for detailed explanation.

The electron distribution measured by SWEA. (a) The field. (b)... Download Scientific
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.

Ions of Transition Elements Mooramo
In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is.

Electron Configuration Of Copper 1+ worksheet
Distribution of Electrons in Shell in Cu Atom 4. Valence Electrons in Cu 5. Valency of Cu Key Takeaways FAQs 1. Copper- Cu Copper (Cu) is a metallic element with the atomic number 29, located in group 11 and period 4 of the periodic table. It has a reddish-orange color and is an excellent conductor of electricity and heat.

Copper Signs of Copper Deficiency Good Whole Food
Element Copper (Cu), Group 11, Atomic Number 29, d-block, Mass 63.546.. Members of a group typically have similar properties and electron configurations in their outer shell.. (very low risk) to 10 (very high risk). This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration.

Electron Configuration for Copper (Cu, Cu+, Cu2+)
Copper, silver, and gold are in group 11 of the periodic table; these three metals have one s-orbital electron on top of a filled d- electron shell and are characterized by high ductility, and electrical and thermal conductivity.