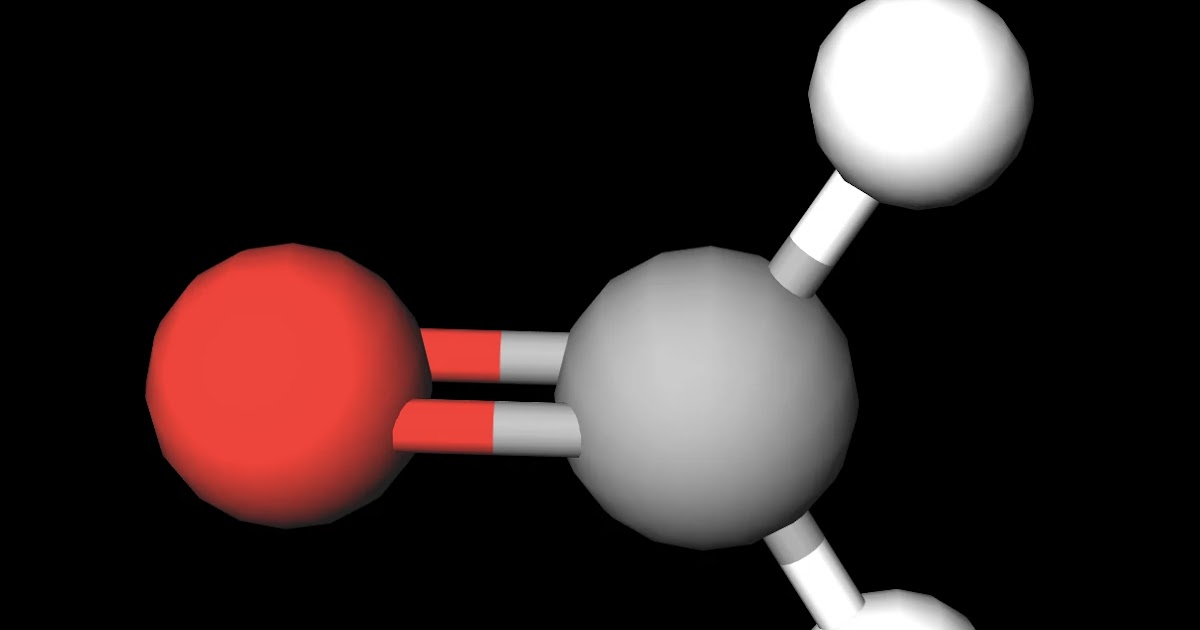
Is O2 Polar Or Nonpolar?
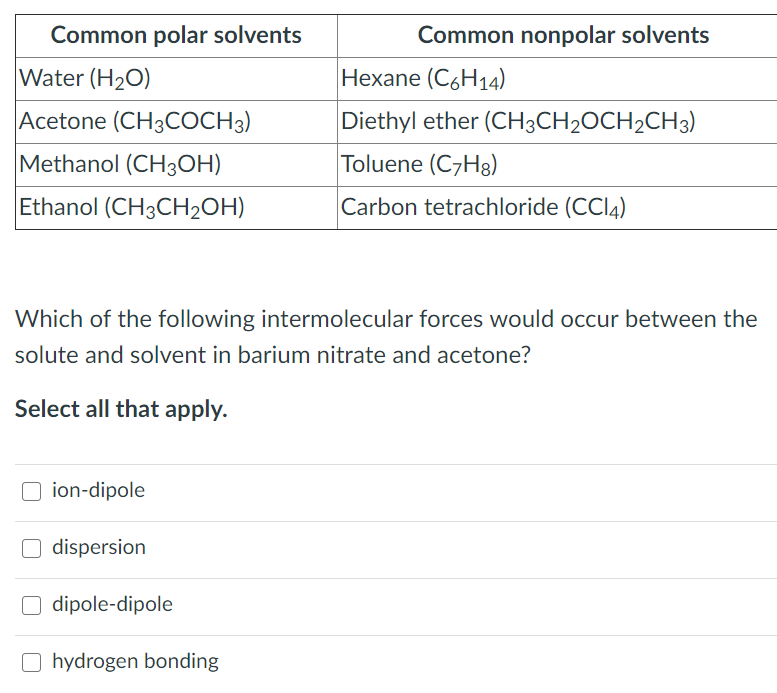
Several intermolecular bonds exist and these include: 1) Hydrogen bonds - the strongest; formed between polar molecules 2) London dispersion forces (LDF) - the weakest; formed between nonpolar molecules 3) Ion-dipole bonds - formed between polar and ionic compounds Personally CH4 should not be included in the list because it is naturally in the.

Is oxygen gas polar or nonpolar? Gek Buzz
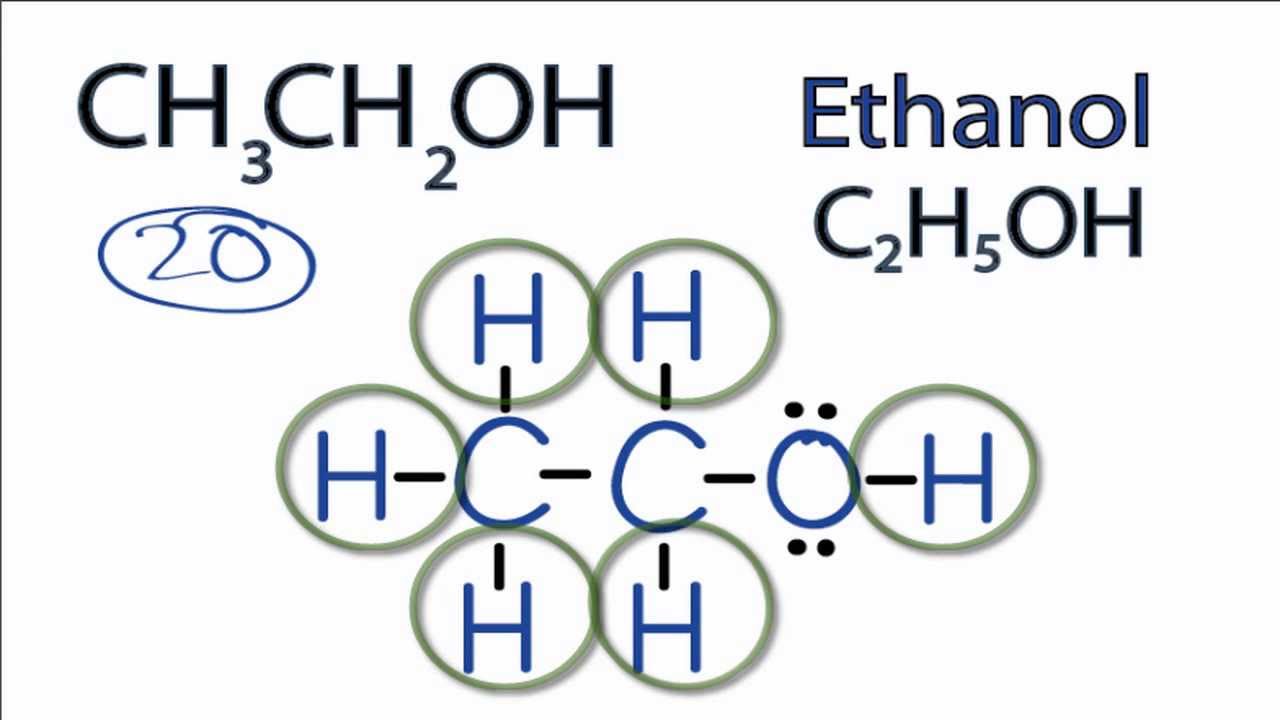
Chemistry Chemistry questions and answers 4. Compound: CH3CH2OH (Ethanol) Lewis Structure 3-D Structure Skeletal Structure Functional Group (s) Present: Central Atom (s) Geometry: Polar or Non-Polar 5.

SOLVED CH3CH2OH has a much higher boiling * 3 points point than CH3O
Helium is nonpolar and by far the lightest, so it should have the lowest boiling point. Argon and N 2 O have very similar molar masses (40 and 44 g/mol, respectively), but N 2 O is polar while Ar is not. Consequently, N 2 O should have a higher boiling point. A C 60 molecule is nonpolar, but its molar mass is 720 g/mol, much greater than that.

Is H2O polar or nonpolar and why? YouTube
H H ** ** ** H : C : C : O : H ** ** ** H H Q3. Give the electron pair and molecular geometries for each central atom. Q4. Give the bond polarity for each type of bond in the molecule. = There are two types of bonds in ethanol. The C-H bonds are nonpolar covalent. Q5. Is your molecule polar or nonpolar ? = Ethanol is both polar and nonpolar. Q6.

Is CH3CH2OH Polar or Nonpolar? Polarity of Ethanol
Helium is nonpolar and by far the lightest, so it should have the lowest boiling point. Argon and N 2 O have very similar molar masses (40 and 44 g/mol, respectively), but N 2 O is polar while Ar is not. Consequently, N 2 O should have a higher boiling point. A C 60 molecule is nonpolar, but its molar mass is 720 g/mol, much greater than that.