
N3 (Azide Ion) Molecular Geometry, Bond Angles & Electron Geometry
Chemical Bonding: N 3 Lewis Structure Drawing the Lewis Structure for N 3- Viewing Notes: There are a total of 16 valence electrons in the N 3- Lewis structure. With N 3- you'll need to form two double bonds between the Nitrogen atoms to fill the octets and still use only the 34 valence electrons available for the molecule.
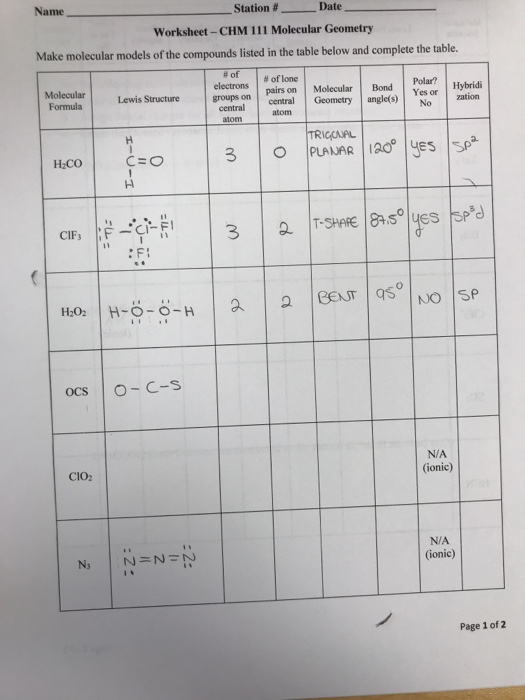
Solved Make molecular models of the compounds listed in the
Written by Priyanka in Lewis Structure The chemical formula N3- represents the Azide ion. The Azide ion is a conjugate base of Hydrazoic acid (HN3). It is composed of three Nitrogen atoms and can have multiple resonance structures.

NO3 Molecular Geometry / Shape and Bond Angles YouTube
Azide. The azide anion. In chemistry, azide ( / ˈeɪzaɪd /, AY-zyd) is a linear, polyatomic anion with the formula N− 3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. [1] The dominant application of azides is as a.

11333.jpg
D With two nuclei around the central atom and one lone pair of electrons, the molecular geometry of SnCl 2 is bent, like SO 2, but with a Cl-Sn-Cl bond angle of 95°. The molecular geometry can be described as a trigonal planar arrangement with one vertex missing. Exercise. Predict the molecular geometry of each molecule. SO 3; XeF 4.

Hybridization, Molecular Geometry and Bond Angles without/with lone
Geometry of Molecules. Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity.

N3 Lewis Structure How to Draw the Lewis Structure for N3 YouTube
Describe the molecular geometry of N 3 −. Molecular geometry: There are five electron-pair geometries: linear, trigonal planar, octahedral, tetrahedral, and trigonal bipyramidal.

AsF3 Molecular Geometry Science Education and Tutorials
In the N 3- Lewis structure, there are two double bonds around the nitrogen atom, with two other nitrogen atoms attached to it, and on the left and right nitrogen atoms, there are two lone pairs. Also, there is a negative (-1) charge on the left and right nitrogen atoms, and a positive (+1) charge on the center nitrogen atom.

Is NO3 Polar or Nonpolar? Techiescientist
Chemistry Chemistry questions and answers 4. Using the Lewis dot structure, predict the molecular geometry of the following and state the hybridization and bond angle of the central atom. a. N3- b. NO3- c. BF4- d. CH4 e. C2H2 This problem has been solved!
Top N3 Molecular Geometry Pics GM
481 149K views 10 years ago NO3- Lewis, Resonance, Shape, Formal Charges, and more. For the NO3- Lewis structure we can see that there are three Oxygen atoms around the central Nitrogen (N) atom..

Lone Pair of Electrons
D With two nuclei around the central atom and one lone pair of electrons, the molecular geometry of SnCl 2 is bent, like SO 2, but with a Cl-Sn-Cl bond angle of 95°. The molecular geometry can be described as a trigonal planar arrangement with one vertex missing. Exercise. Predict the molecular geometry of each molecule. SO 3; XeF 4.

Chemistry Lifeboat Molecular Geometry
21K views 3 years ago An explanation of the molecular geometry for the N3 - ion (Azide Ion) including a description of the N3 - bond angles. The electron geometry for the Azide Ion is.

Nitride Ion
Nitrogen is a group VA element in the periodic table and contains five electrons in its last shell. To find out total valence electrons given by a particular element, you should multiply number of electrons of the valance shell by the number of atoms of that element in respective molecule. valence electrons given by nitrogen atoms = 5*3 = 15

CH3NH2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Description Azide anion is a pseudohalide anion. It has a role as a mitochondrial respiratory-chain inhibitor. It is a conjugate base of a hydrogen azide. ChEBI Organic or inorganic compounds that contain the -N3 group. Medical Subject Headings (MeSH) 1 Structures 1.1 2D Structure Structure Search Get Image Download Coordinates

Answered Give the electrondomain and molecular… bartleby
The geometry of BCl3 BCl 3 is also given in Figure 7.2: it is trigonal planar, with all four atoms lying in the same plane, and all Cl−B−Cl Cl − B − Cl bond angles equal to 120o 120 o. The three Cl Cl atoms form an equilateral triangle. The Boron atom has only three pairs of valence shell electrons in BCl3 BCl 3.

The electron pair geometry and shape around C2 and N3 in the molecule
Azide [N3]- ion Lewis structure, molecular geometry or shape, resonance structure, polar or non-polar, hybridization, bond angle N 3- is the chemical formula for the azide ion, also known as hydrazoate. It is an anion composed of three nitrogen (N) atoms. It is the conjugate base of hydrazoic acid/ hydrogen azide (HN 3 ).

Molecular Geometry of I3 TyrellanceHutchinson
1. N3- Lewis Structure: Here's a step-by-step guide on drawing the N3 - Lewis structure. Step 1: draw sketch • To begin, count the total amount of valence electrons. Nitrogen is in group 15 of the periodic table. As a result of this, nitrogen has five valence electrons. Because N 3- contains three nitrogen atoms,